Once again Kashmir talent has been acknowledged at international level. The US Food and Drug Administration (FDA) has granted premarket notification clearance for two catheters invented by Kashmiri American doctor, Riyaz Bashir (MD, FACC, RVT). The catheters are first of its kind and are expected to revolutionize the clot management.
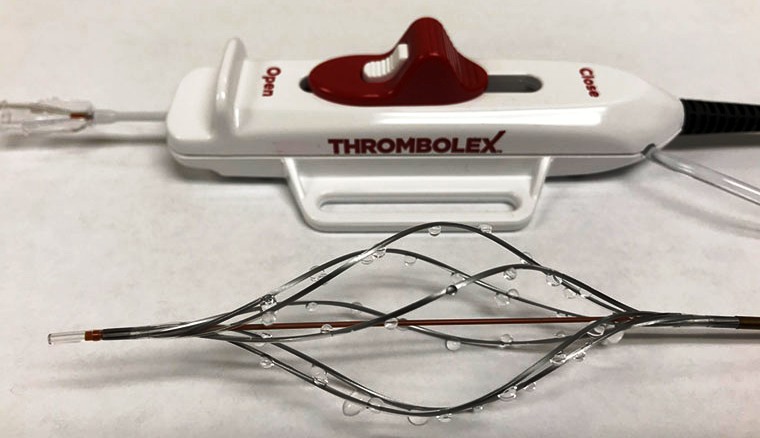
The two catheters developed by Kashmir doctor Riyaz Bashir got FDA clearance for us.
A Professor of Medicine at the Lewis Katz School of Medicine at Temple University (LKSOM) and Director of Vascular and Endovascular Medicine at Temple University Hospital, and Nicholas Green, Director of Research & Development at Thrombolex, the devices would be helpful in managing the cardiac interventions, especially the clot management, better.
The catheters are technically owned by the Thrombolex, a company founded in partnership with Temple University to develop these catheter-based clot dissolving devices. This clearance now allows Thrombolex to commercialize the catheters.
“The Bashir™ Endovascular Catheter (BEC) is cleared for the controlled and selective infusion of fluids, including clot-dissolving medications, into the veins and arteries of the peripheral vasculature,” a Temple University post said. “The BEC is unique because it’s the only catheter of its kind that, once advanced into the clot, can be expanded by the physician into six expandable mini-catheters to deliver medications in precise locations throughout the cross-section of the clot.”
“The Bashir N-X™ Endovascular Catheter (BEC N-X) is cleared for the controlled and selective infusion of fluids chosen by the physician into both the peripheral and pulmonary vasculature, which is comprised of the blood vessels of the lungs. Unlike the BEC, the BEC N-X is not expandable.”
“My inspiration for the BEC platform technology was to develop a device that I hoped would provide better treatment outcomes by rapid restoration of blood flow through the blood clot thereby enhancing the breakdown of the clot,” Dr Bashir was quoted saying by the Temple University post.
“Acute Venous Thromboembolic (VTE) disease, which is marked by blood clots that start in a vein – often in the deep veins of the leg, groin or arm – and can break off and travel to the lungs causing a pulmonary embolism, has become a significant public health concern in the US. Approximately 900,000 patients have been diagnosed with VTE and it causes up to 100,000 deaths each year, according to the CDC.”
“Our dedicated team is proud to have received FDA clearance for these two unique catheter-directed thrombolysis products to be used in the treatment of patients suffering from acute VTE disorders,” the post quoted Marvin Woodall, Chairman and CEO and co-founder of Thrombolex, saying.
Thrombolex has also received FDA approval to begin a multicenter early feasibility study in the clinical setting to evaluate the safety and feasibility of the Bashir™ Endovascular Catheter in the treatment of acute pulmonary embolism.
The post has mentioned that the inventor, Dr Bashir, has an equity interest in Thrombolex, Inc. a medical device company developing interventional catheter-based therapies for the rapid and effective treatment of acute venous thromboembolic disorders. Besides, Temple University also holds a financial interest in Thrombolex, Inc.